Data Sharing Process
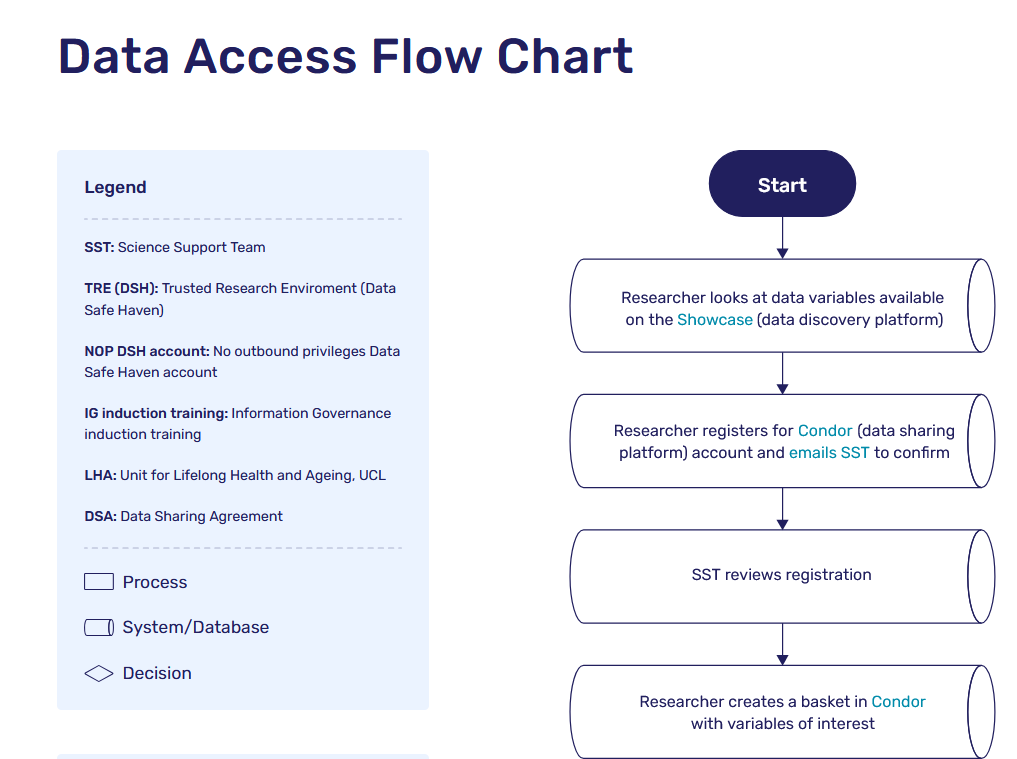
Flow chart of data sharing process
To find out the process for data sharing, check out the data sharing flow chart.
The review process
All proposals are considered by members of the LHA/NSHD Data Sharing Committee and by specialist reviewers if necessary. Proposals are considered by the LHA/NSHD Data Sharing Committee on a regular basis.
As a general guideline, successful research proposals meet the following criteria:
- No breach of confidence should be incurred
- The use of the data should be within the bounds of consent given by cohort members, and comply with MRC guidance on ethics and research governance
- There should be no risk to the viability or reputation of the study from use of the data supplied
- The proposal should be from bone fide researchers working within an MRC recognised organisation who have the necessary expertise to undertake the project. In the case of students applying for access, their supervisor must be a co-applicant and ensure that appropriate academic and data security guidance are adhered to at all times
Researchers are encouraged to view the list of previously approved data sharing proposals to assess points of similarity or overlap with already existing projects.
The LHA/NSHD Data Sharing Committee reserves the right to prioritise applications where necessary. Projects that will incur significant extra work by LHA data management staff may be subject to a charge to cover the additional costs incurred by the LHA. Any such costs will be notified in advance.
There is more information avaliable on the principles, processes and parameters of data sharing in LHA.
Correspondence and timings for review of submissions
Receipt of your application will be notified using the email address you supplied. If you have not received an electronic confirmation of your submission within 10 working days please contact us.
The LHA/NSHD Data Sharing Committee meets regularly to assess all new applications to use NSHD data. In normal circumstances a decision will be taken by the LHA/NSHD Data Sharing Committee within two weeks of receipt of the proposal, however this is dependent upon the timing of the original proposal in relation to the meetings. Applicants will be notified by email if their application to use the data will be delayed beyond these time lines.
Facilitated collaborations
Facilitated collaborations are required in the following circumstances and may involve additional conditions for use of the data: this list is indicative rather than exhaustive.
- Proposals that require access to a specialist database created by a key collaborator or have required significant investments in time by the study team
- Proposals that require access to sensitive data. Sensitive data include, but are not limited to, the cause and date of death of a study member, data on residential or emigration history, cancer registration information or other data obtained from NHS Digital, and medical and other information with small cell counts (e.g. some ICD hospital admissions, or childhood enuresis variables)
- Proposals requiring access to data or meta-data that have not yet been through vigorous quality assurance protocols in place and will require a significant amount of time to check and/or clean Proposals requiring access to extensive biomedical data
Rejected proposals and appeals procedure
In the unusual situation that the LHA/NSHD Data Sharing Committee cannot approve a proposal, the external researcher can appeal, in the first instance to the Director of the LHA. The Director may decide to grant the appeal or to refer it to an external independent body. Full details of the appeals procedure will be supplied to the applicants should this prove necessary.
Information Governance
The LHA has robust governance mechanisms to ensure that all our activities meet the highest standards. Our Senior Management Team and Science Support Team ensure that:
- Unit activities meet the highest standards of ethical practice in epidemiological research
- Ethical, scientific, financial, health and safety and intellectual property matters are properly executed under the leadership and management of the Director
- Policies and procedures are in place to safeguard the reputation of the study, the interests of study members and the security of the data
- The scientific potential of NSHD is maximised through the provision of scientific advice to the team and their collaborators and by ensuring that policies and procedures are in place that facilitate data discovery and use by bona fide researchers
- The potential for NSHD research findings to improve human health is maximised
Citing NSHD Datasets
The NSHD now has a set of persistent identifiers (DataCite DOIs, or just DOIs) that allow the tracking of research datasets as well as publications. It is important that all authors cite these DOIs in publications based on NSHD data.
